Research
Discover the main research lines of Pharmamel Drug Discovery.
Melatonin research
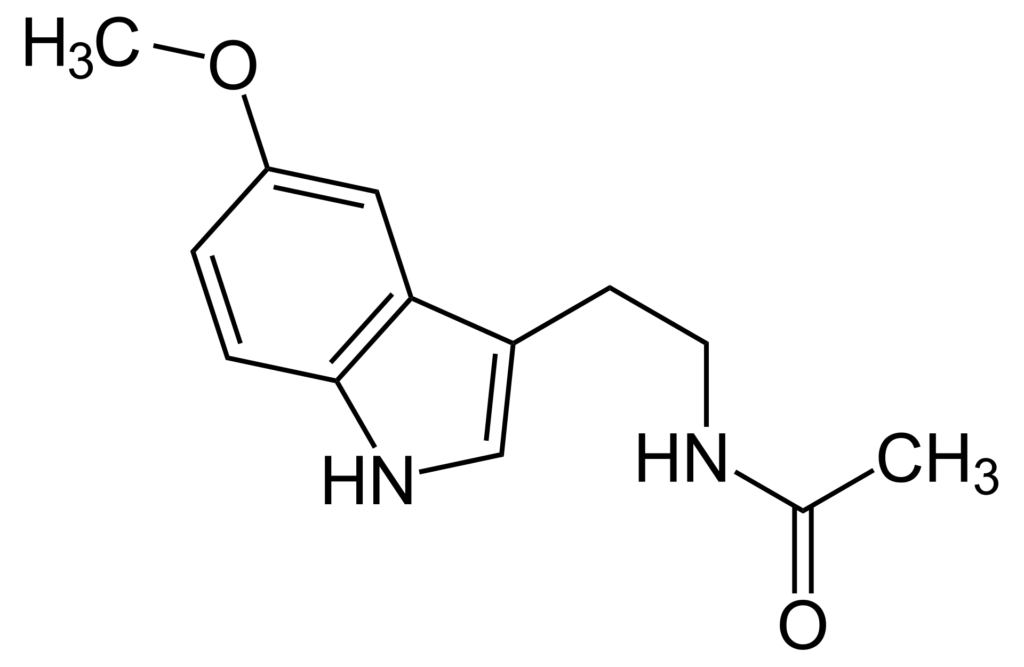
Melatonin is a molecule produced in all living organisms, both animal and plant, with unique functions that regulate homeostasis.
It is produced in the pineal gland of the brain and is secreted into the cerebral and systemic circulation, and its synthesis is regulated by the photoperiod through a complex polysynaptic pathway from the retina. During the day, white light blocks its production, which is why there is virtually no circulating melatonin in the blood. However, from dusk, as the light becomes warm as the sun sets, its synthesis begins so that 4-5 hours later there are sufficient levels to induce the drowsiness that leads to the onset of sleep.
But there is another kind of melatonin, called extrapineal melatonin, which is produced in all the organs and tissues of the body, not controlled by the photoperiod and produced on demand depending on the state of the cell. Although it is structurally the same molecule as pineal-origin melatonin, its functions are very different because it is produced inside the cell at much higher concentrations than pineal melatonin. At these concentrations, melatonin has very potent properties as an antioxidant, anti-inflammatory, and enhancer of mitochondrial function.
Pathologies
Indeed, the previously mentioned properties of melatonin establish its clinical application. On one hand, the use of pineal melatonin as a chronobiotic at low doses is particularly useful in circadian sleep disorders as well as other dysfunctions including seasonal affective disorder and similar conditions.
On the other hand, what is of more interest here, are the antioxidant, anti-inflammatory, and mitochondrial activation properties that make it especially useful at high doses in the clinic for pathologies that involve oxidative damage, inflammatory activation, and mitochondrial dysfunction.
Among these applications of melatonin, we include neurodegenerative diseases, cancer, infectious diseases such as COVID-19, and other viral pathologies, acute inflammatory diseases like sepsis, and aging itself.
Clinical Trials
Over the more than 35 years that we have been working with melatonin, we have developed various experimental models in cell cultures, zebrafish, mice, and rats to evaluate the efficacy, dosages, and potential side effects of melatonin, so as to apply it in human clinical settings.
Clinical Trial in Sepsis
Initially, we conducted appropriate preclinical studies in various models of sepsis, evaluating the use of a melatonin injectable to reduce inflammation, counteract septic shock and multi-organ failure, and analyze the effects in terms of mortality and survival, as well as undesirable treatment effects. The results have been very clear: injected melatonin recovers animals from septic shock and multi-organ failure, and reduces mortality. Additionally, toxicity analyses of melatonin demonstrated not only a total absence of toxicity but also normalized hepatic, renal, cardiovascular, and metabolic functions affected by sepsis.
These results, published in various international journals (see documents), encouraged us to conduct a clinical trial in sepsis.
Why in sepsis? Sepsis is an acute inflammatory process as a result of a primarily bacterial or viral systemic infection, characterized by an exacerbated activation of innate immunity that causes hypovolemic shock, tissue hypoperfusion, multi-organ failure, and risk of death. Indeed, the mortality rate from sepsis exceeds 25% of patients admitted to ICUs worldwide, increasing by 1.5% annually, among other reasons, due to antibiotic resistance. There are about 50 million cases of sepsis globally, with 11-15 million deaths and 19 million survivors who are left with significant sequelae for the rest of their lives. Nearly half (about 20 million) of sepsis cases occur in children. The cost of sepsis is very high, about €30,000 per patient. All this data motivates the WHO to urge all countries in the world to find a solution to this serious pathology.
From a pathophysiological standpoint, the exaggerated inflammatory response in sepsis causes immense oxidative stress that damages organs, tissues, and the vascular system. On the other hand, the mitochondrial failure that occurs in sepsis reduces the bioenergetic efficiency needed for the body to fight this pathology, hence the high mortality and poor prognosis. Precisely this characteristic triad of sepsis is targeted by melatonin.
Therefore, we applied to the AEMPS to conduct a Phase II clinical trial in sepsis patients in the ICU (EUDRACT 2008-006782-83), to evaluate the efficacy of intravenous melatonin (Pharmamel’s patent) for treating this disease. The results indicated that, at the used dosage, melatonin significantly reduced mortality by more than 15% and hospital stay by 20%. As this was the first clinical trial with an intravenous melatonin injectable, the most important outcome was determining the complete absence of toxicity of this treatment.
Clinical Trial in COVID-19
In 2020, we were preparing for the next stage, a Phase III clinical trial of injectable melatonin in patients with sepsis, when the COVID-19 pandemic occurred. Upon analyzing the pathophysiology of this condition, a process was identified that, in addition to acute respiratory failure, involved an exaggerated acute inflammatory response of innate immunity, hypovolemic shock, and multi-organ failure, along with endothelial damage to the vascular system, which were the causes of the high mortality of these patients in the ICU. In other words, COVID-19 patients were dying from sepsis.
Clinical Trial for Mucositis
After demonstrating the significant efficacy of an orally administered melatonin gel in preclinical animal studies, a Phase II clinical trial (EudraCT no.: 2015-001534-13) was conducted under the regulation and supervision of the Spanish Agency of Medicines and Medical Devices (AEMPS). This trial was done in 84 patients with head and neck cancer undergoing radiotherapy and chemotherapy, cisplatin, or cetuximab, in 12 hospitals in Spain.
The effectiveness and efficacy of this innovative orally administered melatonin drug in the treatment of mucositis were confirmed by the results obtained in the studies, which highlighted the following main aspects:
- Significant decrease in the incidence of mucositis by up to 56%
- Decrease in the duration of mucositis by up to 67%
- Significant decrease in oral and esophageal inflammation
- Decrease in the use of opioids for pain relief
- Decrease in the use of nasogastric tubes
- No associated side effects
Documents and Publications
Clinical Trial in Sepsis
- Autorización AEMPS ensayo clínico SepsisDownload
- Ensayo de fase II Sépsis (Journal of Pîneal Research)Download
- Publicación resultados ensayo clínico SepsisView document
- Publicaciones melatonina y SepsisView document
Clinical Trial in Mucositis
No PDFs found.
Clinical Trial in COVID-19
- Autorización AEMPS ensayo clínico COVID-19Download
- Publicación protocolo ensayo clínico COVID-19 (Fase 2)View document
Other Documents on Melatonin and COVID-19
Collaborations
Throughout these years of research with melatonin, we have established various scientific collaborations with numerous international and national centers, among which the following stand out:
- Prof. Russel J Reiter, Ph.D., Dr. h.c.mult. Professor of Cell Biology, Clarivate Analytics List of Highly Cited Scientists, Department of Cell Systems and Anatomy, Joe R. and Teresa Lozano Long School of Medicine, UT Health San Antonio, Texas, USA.
- Prof. Daniel P Cardinali, Professor of Physiology, Pontificia Universidad Católica Argentina, Faculty of Medical Sciences, Department of Teaching and Research, Buenos Aires, Argentina.
- Prof. Juan Miguel Guerrero Montavez, Professor of Biochemistry and Molecular Biology, University of Seville, Head of the Clinical Analysis Service at Hospital Virgen del Rocío in Seville.